Immunoglobulin E
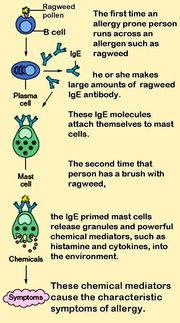
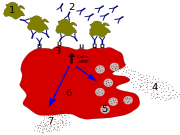
In biology, Immunoglobulin E (IgE) is a class of antibody (or immunoglobulin "isotype") that has only been found in mammals. It plays an important role in allergy, and is especially associated with type 1 hypersensitivity.[1] IgE has also been implicated in immune system responses to most parasitic worms[2] like Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica,[3][4][5] and may be important during immune defense against certain protozoan parasites such as Plasmodium falciparum.[6]
Although IgE is typically the least abundant isotype - blood serum IgE levels in a normal ("non-atopic") individual are only 0.05% of the IgG concentration[7], compared to 10 mg/ml for the IgGs (the isotypes responsible for most of the classical adaptive immune response) - it is capable of triggering the most powerful immune reactions.
IgE was discovered in 1966 by the Japanese scientist couple Teruka and Kimishige Ishizaka.[8]
Contents |
Receptors
IgE elicits an immune response by binding to Fc receptors found on the surface of mast cells and basophils, and are also found on eosinophils, monocytes, macrophages and platelets in humans. Fc has two types:
- FcεRI, the high-affinity IgE receptor
- FcεRII, also known as CD23, is the low-affinity IgE receptor
IgE can upregulate the expression of both Fcε receptors. FcεRI is expressed only on mast cells and/or basophils in both mice and humans. Aggregation of antigens and binding of IgE to the FcεRI on mast cells causes degranulation and the release of mediators from the cells, while basophils cross-linked with IgE release type 2 cytokines like interleukin-4 (IL-4) and interleukin-13 (IL-13) and other inflammatory mediators. The low affinity receptor (FcεRII) is always expressed on B cells, but its expression can be induced on the surfaces of macrophages, eosinophils, platelets and some T cells by IL-4.
Physiology
There is much speculation into what physiological benefits IgE contributes, and so far, circumstantial evidence in animal models and statistical population trends have hinted that IgE may be beneficial in fighting gut parasites such as Schistosoma mansoni, but this has not been conclusively proven in humans.
Although it is not yet well understood, IgE may play an important role in the immune system’s recognition of cancer[9], in which the stimulation of a strong cytotoxic response against cells displaying only small amounts of early cancer markers would be beneficial. Of course, if this were the case, anti-IgE treatments such as omalizumab might have some undesirable side effects.
Role in disease
Atopic individuals can have up to 10 times the normal level of IgE in their blood (as do sufferers of hyper-IgE syndrome). However, this may not be a requirement for symptoms to occur as has been seen in asthmatics with normal IgE levels in their blood - recent research has shown that IgE production can occur locally in the nasal mucosa[10].
IgE that can specifically recognise an "allergen" (typically this is a protein, such as dust mite DerP1, cat FelD1, grass or ragweed pollen, etc.) has a unique long-lived interaction with its high affinity receptor, FcεRI, so that basophils and mast cells, capable of mediating inflammatory reactions, become "primed", ready to release chemicals like histamine, leukotrienes and certain interleukins, which cause many of the symptoms we associate with allergy, such as airway constriction in asthma, local inflammation in eczema, increased mucus secretion in allergic rhinitis and increased vascular permeability, ostensibly to allow other immune cells to gain access to tissues, but which can lead to a potentially fatal drop in blood pressure as in anaphylaxis. Although the mechanisms of each response are fairly well understood, why some allergics develop such drastic sensitivities when others merely get a runny nose is still one of science's hot topics. Regulation of IgE levels through control of B cell differentiation to antibody-secreting plasma cells is thought to involve the "low affinity" receptor, FcεRII or CD23. CD23 may also allow facilitated antigen presentation, an IgE-dependent mechanism whereby B cells expressing CD23 are able to present allergen to (and stimulate) specific T helper cells, causing the perpetuation of a Th2 response, one of the hallmarks of which is the production of more antibodies.
Pharmacology
IgE may be an important target in treatments for allergy and asthma.
Currently, severe allergy and asthma is usually treated with drugs (like anti-histamines) that damp down the late stages of inflammation and relax airway smooth muscle. Unfortunately, these treatments are fairly broad in their action, and so many have unpleasant side effects; they may also inhibit important protective responses.
In 2002, researchers at The Randall Division of Cell and Molecular Biophysics determined the structure of IgE[11]. Understanding of this structure (which is atypical of other isotypes in that it is highly bent and asymmetric), and of the interaction of IgE with receptor FcεRI will enable development of a new generation of allergy drugs that seek to interfere with the IgE-receptor interaction. A new treatment, omalizumab, a monoclonal antibody, recognises IgE not bound to its receptor and is used to neutralise or mop-up existing IgE and prevent it from binding to cells. It may be possible to design treatments cheaper than monoclonal antibodies (for instance, small molecule drugs) that use a similar approach to inhibit IgE binding to its receptor.
In 1975 Robert N. Hamburger, M.D. published "Peptide Inhibition of the P-K Reaction" based on blocking up to 89% of the IgE receptors on mast cells by the pentapeptide representing amino acids 320 to 324 on the epsilon chain of IgE.[12]
See also
- Antibodies
- IgM, IgA, IgD, IgG
References
- ↑ Gould H et al. (2003). "The biology of IGE and the basis of allergic disease". Annu Rev Immunol 21: 579–628. doi:10.1146/annurev.immunol.21.120601.141103. PMID 12500981.
- ↑ Erb KJ (2007). "Helminths, allergic disorders and IgE-mediated immune responses: where do we stand?". Eur J Immunol 37 (5): 1170–1173. doi:10.1002/eji.200737314. PMID 17447233.
- ↑ Fitzsimmons C, McBeath R, Joseph S, Jones F, Walter K, Hoffmann K, Kariuki H, Mwatha J, Kimani G, Kabatereine N, Vennervald B, Ouma J, Dunne D (2007). "Factors affecting human IgE and IgG responses to allergen-like Schistosoma mansoni antigens: Molecular structure and patterns of in vivo exposure". Int. Arch. Allergy Immunol. 142 (1): 40–50. doi:10.1159/000095997. PMID 17019080.
- ↑ Watanabe N, Bruschi F, Korenaga M (2005). "IgE: a question of protective immunity in Trichinella spiralis infection". Trends Parasitol. 21 (4): 175–8. doi:10.1016/j.pt.2005.02.010. PMID 15780839.
- ↑ Pfister K, Turner K, Currie A, Hall E, Jarrett EE (1983). "IgE production in rat fascioliasis". Parasite Immunol 5 (6): 587–593. doi:10.1111/j.1365-3024.1983.tb00775.x. PMID 6657297.
- ↑ Duarte J, Deshpande P, Guiyedi V, Mécheri S, Fesel C, Cazenave P, Mishra G, Kombila M, Pied S (2007). "Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status". Malar. J. 6: 1. doi:10.1186/1475-2875-6-1. PMID 17204149.
- ↑ Arch Pathol Lab Med. 2000 Sep;124(9):1382-5. Immunoglobulin E: importance in parasitic infections and hypersensitivity responses. Winter WE, Hardt NS, Fuhrman S. PMID: 10975945
- ↑ Ishizaka K, Ishizaka T, Hornbrook MM (1966). "Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity". J. Immunol. 97 (1): 75–85. PMID 4162440.
- ↑ Karagiannis S et al. (2003). "Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells". Eur J Immunol 33 (4): 1030–40. doi:10.1002/eji.200323185. PMID 12672069.
- ↑ Takhar P et al. (2005). "Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis". J Immunol 174 (8): 5024–32. PMID 15814733.
- ↑ Wan T et al. (2002). "The crystal structure of IgE Fc reveals an asymmetrically bent conformation". Nat Immunol 3 (7): 681–6. doi:10.1038/ni811. PMID 12068291.
- ↑ Hamburger R (1975). "Peptide inhibition of the Prausnitz-Küstner reaction". Science 189 (4200): 389–90. doi:10.1126/science.1145208. PMID 1145208.
|
|||||||||||||||||||||||||||||||||||||
|
||||||||||||||